Samples of the stopper rods for continuous casting ladles produced by a certain factory were analyzed, examining the structure and composition of the rod body, rod tip, oxidation-resistant coating, and anti-adhesion coating. Results indicate: The outer layer of the stopper head contains a higher proportion of granular aggregates and exhibits a denser structure than the core. The gradient distribution in the head material enhances erosion resistance. The anti-adhesion coating composition includes Al₂O₃, SiO₂, P₂O₅, Na₂O, and K₂O. The coating exhibits low adhesion strength to the substrate and is prone to peeling. The anti-oxidation coating of the plug rod consists of Na₂O, Al₂O₃, SiO₂, K₂O, TiO₂, and CaO, with a high proportion of alkali metal R₂O phases that readily form low-melting-point glazes at elevated temperatures. The inclusions in the silicon steel may originate from erosion products of the anti-adhesion coating, anti-oxidation coating, or the plug rod tip.
The stopper rod is used to control the flow velocity of molten steel between the ladle and the mold, primarily employed in the production of high-quality steel and alloy steel [1]. During operation, the rod tip is the most critical component, determining both flow control effectiveness and the rod’s service life [2]. Erosion and abrasion from molten steel cause deformation of the rod tip; The worn-away sections create vortices with the upper bowl section of the nozzle, narrowing the flow path and increasing contact area. In the later stages of use, erosion deformation widens the gap between the plug head and the nozzle bowl, increasing the required drop height for closure and degrading flow control until failure. Erosion products often form inclusions carried into the mold, ultimately compromising steel quality.
Plug materials include aluminum-carbon and aluminum-zirconium-carbon types, with service life primarily determined by erosion at the slag line. An optimal anti-oxidation effect is achieved when the surface oxide coating forms a glassy, non-porous glaze. If the heating process produces a porous glaze, oxygen can permeate and react with carbon within the plug, causing oxidation [3]. Calcium-treated low-alloy high-strength steels contain high calcium levels, reacting with aluminum-carbon plug rods to form low-melting-point silicates and calcium aluminates, accelerating erosion. Switching to magnesium-carbon rod tips mitigates abnormal erosion, extending ladle life by 2–6 consecutive pours [4]. Anti-oxidation coatings for aluminum-carbon and aluminum-magnesium-carbon stopper rods often incorporate boron-containing materials as flux agents, blended with additives like clay, high-alumina clinker, SiC, and quartz [5]. These coatings form a glassy protective film at temperatures above 800°C. However, some studies suggest that boron oxides form a liquid phase that fills the pores of carbon-containing refractories, thereby reducing carbon oxidation. Excessive use of boron-based compounds as antioxidants can lead to a decline in the mechanical properties of the coating [6]. In practice, few studies systematically analyze the composition, structure, and issues related to oxidation-resistant and anti-adhesion coatings used in plugs from an application perspective. This paper examines the composition, structure, properties, and associated issues of plugs used in the production of non-oriented high-grade silicon steel through dissection analysis.
1. Sampling and analysis of stopper rods for continuous casting tundish
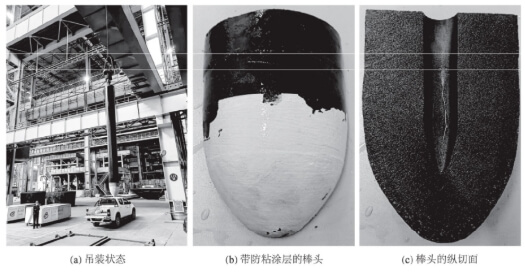
As shown in Figure 1a, the plug rod has a total length of 1,570 mm, with a 120 mm section coated with anti-adhesion paint. The wrist section features a large-end diameter of 135 mm and a small-end diameter of 130 mm. The chemical composition of the cylindrical section of the ramrod is as follows: w(Al₂O₃) = 59.69%, w(SiO₂) = 10.40%, w(CaO) = 0.17%, w(MgO) = 0.10%, w(TFe) = 1.38%, loss on ignition (LOI) = 20.03%. The chemical composition of the plug head is: w(Al₂O₃) = 80.21%, w(SiO₂) = 0.76%, w(CaO) = 0.20%, w(MgO) = 0.12%, w(TFe) = 2.18%, w(C) = 12%, loss on ignition (LOI) = 11.99%. The composition of the rod tip differs from that of the cylindrical section.
As shown in Figure 1b, after cutting the rod, the anti-adhesion coating on the outer surface of the rod tip can be easily scraped off upon contact with water. After drying, the scraped coating was analyzed via powder energy dispersive spectroscopy (EDS) using a field-scanning electron microscope (FSEM), revealing the following composition: w(Al₂O₃) = 64.60%, w(SiO₂) = 26.73%, w(P₂O₅) = 6.71%, w(Na₂O) = 1.38%, w(K₂O) = 0.58%. Based on the composition, the coating is presumed to consist of bauxite, mullite powder, and fine clay particles, blended with a binder such as sodium tripolyphosphate or aluminum dihydrogen phosphate. The coating exhibits minimal strength and softens upon water exposure, indicating it serves as a single-use protective layer likely composed of a thermosetting material.
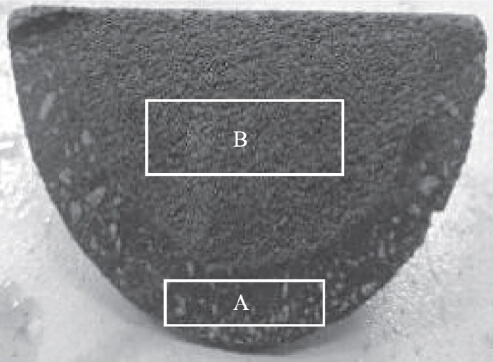
As shown in Figure 1c, longitudinal sectioning of the plug along its length reveals distinct material variations at the plug head: the outermost layer (15–25 mm thick) contains coarse aggregate particles, including white granular aggregate, while the core shows no discernible aggregate. A close-up of the plug rod’s cross-section is shown in Figure 2. Analysis of the outer layer material from section A reveals a bulk density of 2.85 g/cm³ and an apparent porosity of 12.8%. Analysis of the core material from section B shows a bulk density of 2.86 g/cm³ and an apparent porosity of 14.1%. The material exhibits gradient layering, likely designed to enhance erosion resistance, while the straight cylindrical section at the rod tip shows no layering.
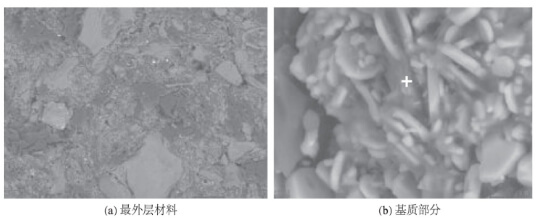
The structure of the outermost layer of the rod head material is shown in Figure 3. The large and small aggregates consist of alumina, while the black component is carbon. The surface scan composition of Figure 3a is summarized in Table 1, suggesting its primary composition is corundum and carbon, with minor amounts of SiO₂ and Na₂O. Figure 3b shows the magnified structure of the material matrix, revealing numerous flake-like and fine-grained aggregates. The locally gray amorphous phase at the “+” location has the composition shown in Table 1, where the large and small particles are aluminum oxide and the black component is carbon. It is inferred that the oxidation inhibitor may be metallic aluminum or ultrafine aluminum oxide powder.
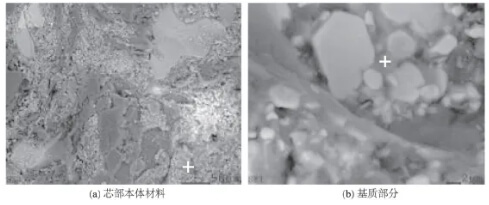
The core structure of the rod head is shown in Figure 4, where the large and small aggregates are composed of alumina, and the black material is carbon. The surface scan composition and the amorphous bonded phase at the “+” location in Figure 4a are summarized in Table 1, primarily consisting of corundum and carbon, with the presence of S and Cl⁻. Based on the analysis of the core material, it is inferred that the alumina content has decreased, the particle size has reduced, and iron impurities are present. Figure 4b shows a magnified section of the rod tip core material. It exhibits numerous elongated and flake-shaped corundum aggregates densely bonded with fine-grained material and an amorphous matrix. The gray amorphous phase at the “+” location, as shown in Table 1, indicates uneven aluminum oxide distribution within the matrix, with locally higher aluminum oxide and lower carbon content. The larger and smaller particles are aluminum oxide, while the black particles are carbon. It is speculated that the oxidation inhibitor may be metallic aluminum or ultrafine aluminum oxide powder.
The temperature of molten steel in the ladle typically ranges from 1,470 to 1,560 °C. To observe the morphological changes of the plug rod at high temperatures, it was heated to approximately 1,000 °C using an oxygen lance combustion method. The surface anti-oxidation coating formed a glaze layer exhibiting localized network-like accumulation with flowing patterns. The composition of the glaze layer formed on the anti-oxidation coating was analyzed using FSEM coupled with energy dispersive spectroscopy (EDS). The surface scan composition of one powder sample was: w(Na₂O) = 9.51%, w(Al₂O₃) = 9.99%, w(SiO₂) = 75.60%, w(K₂O) = 2.20%, w(TiO₂) = 1.05%, w(CaO) = 1.66%. Another powder sample’s surface scan composition was: w(Na₂O) = 8.17%, w(Al₂O₃) = 9.46%, w(SiO₂) = 77.33%, w(K₂O) = 2.31%, w(TiO₂) = 1.20%, w(CaO) = 1.52%. Based on the two sets of compositions, variations in oxide content likely stem from sampling and analytical angle differences. It is inferred that the primary composition of the anti-oxidation coating consists of fused quartz blended with small amounts of clay, sodium silicate, and potassium silicate water glass. The resulting glass structure is relatively dense, effectively blocking gas permeation.
2.Discussion on the Performance, Composition, and Effects on Steel Quality in the Application of Packing Rods
From a usage perspective, the stopper rod is positioned as a functional refractory component. Beyond its high-temperature resistance, erosion resistance, wear resistance, and erosion resistance, its primary function is flow control. Secondly, it prevents erosion or reaction products from entering the molten steel, thereby avoiding the formation of inclusions that could compromise steel properties. This aspect has often been overlooked in the past, as the molten steel controlled by the stopper rod enters the copper mold, which represents the final stage in ensuring molten steel purity. Refractory suppliers focus on plug rod composition, raw material specifications, production conditions, coatings, and baking temperatures—technical indicators primarily related to performance. Conversely, steel mill users rarely scrutinize the plug rod’s material composition itself. Their attention centers on operational effectiveness, and when issues arise, they typically examine the performance metrics provided by the plug rod supplier.
Taking a plug rod produced by a certain factory as an example: its Al₂O₃ mass fraction is ≥55% for the core body and ≥75% for the rod head; its properties include a room-temperature bulk density of ≥2.5 g/cm³ for the core body and ≥2.7 g/cm³ for the rod head; apparent porosity is ≤19% for the core body and ≤21% for the rod head; and the flexural strength at room temperature is ≥6 MPa for both the core body and tip. Since stopper rods are irregularly shaped components, the analysis of their compositional properties is closely related to the sampling location and method. Factors such as the cutting boundaries for tip composition samples, the dimensions of flexural strength test specimens, the sampling location, and the cutting method all influence the analysis results. Therefore, from a quality supervision perspective, the frequency of spot checks for the strength, bulk density, and apparent porosity of stopper rods is relatively low.
Figure 5 shows the morphology and fracture surface of a residual sample after a plug rod breakage during production at a certain factory. Measured from the rod tail toward the head, the fracture location occurred between 840 and 1,100 mm. On-site process investigations could only analyze argon flow rate and backpressure changes during the plug rod operation. For instance, with an argon flow rate of 0.06 L/min and backpressure of 0.3 MPa, it was inferred that the argon holes in the plug rod might have become blocked during pouring. The accumulated gas inside could not be expelled, and the high-temperature molten steel rapidly heated the rod body during pouring. causing the gas to expand violently. The pressure was released at the weakest point of the stopper rod, resulting in fracture. However, during the accident cause investigation, residual samples could not be prepared into standard specimens required for performance testing. The analysis relied solely on inspection data provided by the stopper rod supplier, such as flexural strength, bulk density, and apparent porosity. These differed from the actual performance indicators of the stopper rods used on-site, making it difficult to draw conclusions. Therefore, analyzing the composition and structure of the residual stopper rod samples provides a relatively more objective perspective.
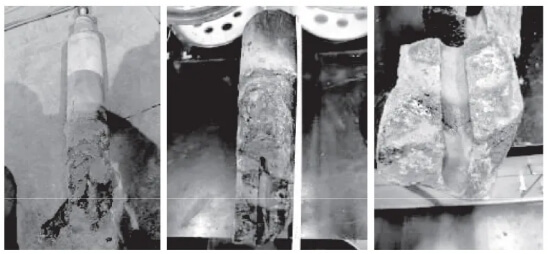
During application, erosion products from the stopper rod may enter the steel, forming fine inclusions that affect material properties. Research indicates that the primary inclusions in silicon steel are AlN, irregular silicate inclusions, and spherical iron oxides and sulfides [7]. Based on the composition of AlN and sulfides, their formation appears unrelated to stopper rod erosion. In non-oriented silicon steel of different grades, 10–20 μm inclusions consist of Al₂O₃ and SiO₂ composite inclusions, though in relatively small quantities. Some are primarily CaO, MgO, Al₂O₃, and SiO₂ composite inclusions. Inclusions measuring 1–10 μm comprise composite inclusions of two or more types, such as CaO, Al₂O₃, SiO₂, and MgO, and are relatively abundant. Inclusions smaller than 1 μm consist of composite inclusions of MnS and MgO or single MnS inclusions, and are also relatively abundant [8]. Scanning electron microscopy reveals inclusions in cast billet specimens [9] comprising CaO, Al₂O₃, SiO₂, and MgO (where SiO₂ and Al₂O₃ are endogenous inclusions in the molten steel, while MgO and CaO are exogenous inclusions). with sizes ranging from 1.2 to 1.5 μm. Larger, complex mixed inclusions are also present, and most composite inclusions exhibit brittleness with surface cracking.
Similar analyses indicate that oxide composite inclusions of CaO, MgO, Al₂O₃, and SiO₂ may originate from slag or from erosion products of the tap plug. Since molten steel enters the mold through the gap between the tap plug and nozzle, slag inclusion formation is unlikely except for potential slag entrainment during late pouring. However, the tap plug itself—including coating and eroded plug head material—can enter the steel stream. Thus, inclusion composition can indicate origin. For example, the anti-adhesion coating composition includes Al₂O₃, SiO₂, P₂O₅, Na₂O, and K₂O, with relatively high Al₂O₃ and P₂O₅ content. If the inclusion composition is similar, it likely originates from peeling of the anti-adhesion coating. The anti-oxidation coating composition includes Na₂O, Al₂O₃, SiO₂, K₂O, TiO₂, and CaO, with relatively high SiO₂ and alkali metal R₂O content. If the inclusion composition is similar, it may originate from molten erosion of the anti-oxidation coating. Additionally, certain steel grades require enhanced erosion resistance at the tap hole tip during smelting, employing MgO-containing carbon materials as the outermost layer. If inclusions closely match the composition of MgO-containing carbon materials, their origin is likely related to erosion at the tap hole tip.
Traceability of inclusions generally involves reverse-engineering the influence of materials from different process stages based on inclusion composition. For instance: – Mg, Si, O-type inclusions may originate from ladle coatings; – Ca, Al, Si, O-type inclusions may originate from ladle cover agents; – Ca, Mg, Al, O-type inclusions may originate from submerged nozzles; Ca, Al, Si, Na, O-type inclusions may originate from the mold protective slag; Ca, Mg, Al, Si, O-type inclusions may originate from ladle casting residues; Cr, Mg, Al, Si, O inclusions may originate from the ladle sand; Al, C inclusions may originate from the ladle stopper. However, in specific process practice, when encountering inclusion issues and implementing corrective measures, if adjusting corresponding equipment or materials fails to resolve the problem, it becomes necessary to address the inclusions by specifically analyzing process variations and corresponding changes, such as examining stopper tips or coatings.
Conclusion
1) Anatomical analysis of the plug rod indicates that its head differs in material composition from the main body. The outer layer of the rod head contains a higher proportion of granular aggregate, with a bulk density of 2.85 g/cm³ and an apparent porosity of 12.8%, making it denser than the core material. This material gradient distribution serves to enhance the material’s erosion resistance.
2) The anti-adhesion coating on the plug head comprises Al₂O₃, SiO₂, P₂O₅, Na₂O, and K₂O, with a high Al₂O₃ content. The coating exhibits low adhesion to the substrate and can be easily scraped off when exposed to water. The anti-oxidation coating on the plug rod comprises Na₂O, Al₂O₃, SiO₂, K₂O, TiO₂, and CaO, with higher SiO₂ and alkali metal R₂O content. The predominant alkali metal R₂O phase readily forms low-melting-point glazes at elevated temperatures.
3) Inclusions in steel may originate from erosion products of anti-adhesion coatings, anti-oxidation coatings, or plug/rod heads. Identification requires compositional analysis of the inclusions.
